Business Need
HIV-1 reverse transcriptase (HIV-1 RT) is the target for a majority of HIV-drugs. The rapid emergence of drug-resistant variants of HIV-1 RT has limited the efficacy of anti-AIDS treatments.
Beactica set out to identify novel scaffolds for inhibition of wild type and drug-resistant HIV-1 RT, with the ultimate aim to develop novel HIV therapeutics for patients in need.
Technical Challenges
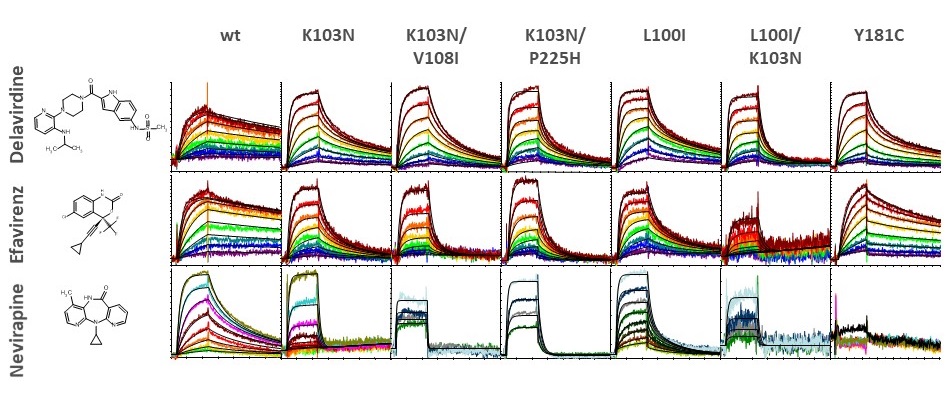
Development of resistance through viral evolution has resulted in the need to consider many variants of HIV-1 RT when sceening. New inhibitors neet to be efficacious against many mutants forms. The image below illustrates the diverse interaction profiles observed between HIV-1 RT variants and three non-nucleoside reverse transcriptase inhibitors (NNRTI).
Solution
Beactica’s team of highly skilled scientists applied the Company’s SPR biosensor-based discovery platform with an efficient experimental design involving multiple key resistance variants of HIV-1 RT whilst also managing other technical challenges.
Results
This pioneering study identified novel allosteric scaffolds with defined modes of action and desirable resistance profiles against key mutant forms of HIV-1 RT. The work also contributed towards improved design and analysis of fragment-based screens targeting proteins with flexible binding sites.
The therapeutic programme was reprioritized by Beactica and the story was published in Journal of Medicinal Chemistry in 2011 [1][2].
HIT-1 RT is a highly flexible protein.
In addition to larger domain movements, there is a conformational change required to open up the NNRTI binding site. This is illustrated in the picture above that shows two different crystal structures of the NNRTI binding site.
Finally, more than 800 NNRTIs had been published. Achieving chemical novelty whilst also overcoming the protein-based challenges was a major hurdle.
References
[1] Geitmann, M., Elinder, M., Seeger, C., Brandt, P., de Esch, I. and Danielson, U.H., Identification of a novel scaffold for allosteric inhibition of wild type and drug resistant HIV-1 reverse transcriptase by fragment library screening. J Med Chem, 2011, 54:699–708, doiDOI: 10.1021/jm1010513.
[2] Brandt, P., Geitmann, M. and Danielson, U.H., Deconstruction of Non-Nucleoside Reverse Transcriptase Inhibitors of Human Immunodeficiency Virus Type 1 for Exploration of the Optimization Landscape of Fragments, J Med Chem, 2011 54:709-718, doi: 10.1021/jm101052g.
Stay Updated
Sign up for the Beactica newsletter to receive our latest news and updates
